Which of the Following Elements Has the Greatest Metallic Character
This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. Some elements which shows metallic character are listed below.

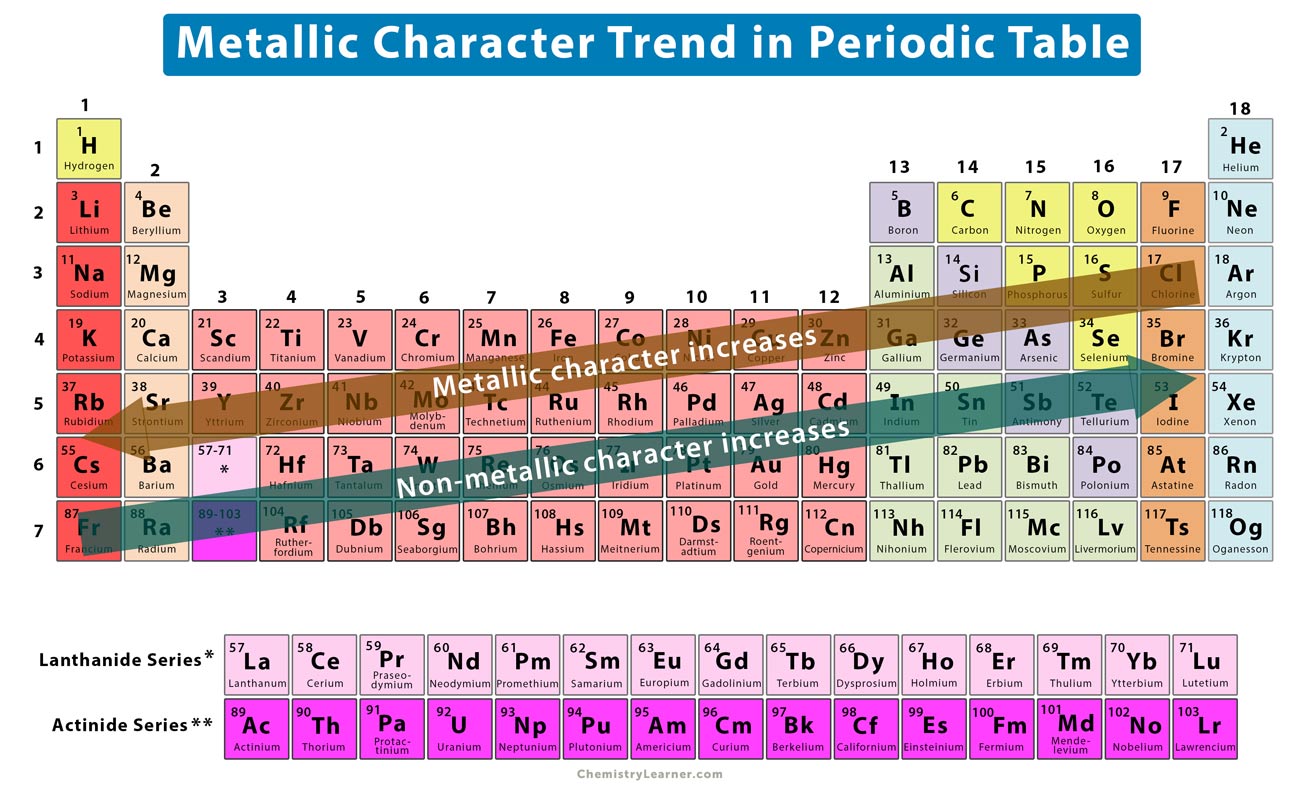
Metallic Character Trend On The Periodic Table
Metallic character increases as you move down an element group in.
. As Calcium and magnesium both belongs to the same group of the periodic table that is group 2. Franium element with highest metallic character caesium next highest level of metallic character sodium. Which of the following atoms has the least metallic character.
That in a copper penny there is one atom for every person on earth. In periods the metallic character decreases when atomic number increases. N O DCI OUESTION 11 QUESTION 11 Which of the following elements has the highest first ionization energy.
Most beryllium compounds BeH2 and beryllium halides such as BeCl2 and some magnesium compounds MgH2 for example are molecular rather than ionic in nature. Advertisement Advertisement Jakedutton7 Jakedutton7 Answer. The answer is no.
Metallic character increases form right to left across a period on the periodic table and from top to bottom down a group. Bismuth Bi Across the period from left to right metallic character decreases meaning when it is going down the last element in a group will have the most metallic character. Move on to Figure 3 and Figure 2.
Answered Aug 16. That atoms are very large d. Au QUESTION 10 Which of the following atoms is the smallest.
That atoms are indivisible b. That atoms are very small c. Atoms of metallic elements tend to 1 gain electrons and form negative ions 2 gain electrons and form positive ions 3 lose electrons and form negative ions 4 lose electrons and form positive ions.
The element in Period 3 that has the greatest metallic character is magnesium. This metallic character depicts the reactivity series of the metals. Asked Aug 16 2019 in Chemistry by Khamseen.
Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. Which of the following elements has the greatest metallic character. The metallic character increases from right to left along a period and top to bottom in a group.
Answered Aug 16 2019 by opking. The alkali metals in group 1 are the most active metals and cesium is the last element in the group for which we have experimental data. Cesium is further down and more left than strontium so cesium will have the most natural metallic character and strontium will have the second highest metallic character.
Since Bismuth bi is the last element it is the most metallic. Which of the following elements has the greatest metallic character. These two atoms have two valence electrons thus they have similar chemical properties.
Metallic character decreases as you move across a period in the periodic table from left to right. Which of the following atoms has the greatest metallic character. Bismuth is the element in group 15 that has the strongest metallic character.
The metallic character increases from top to bottom. Group 15 of the periodic table contains five elements beginning with. Which of the following elements has the greatest metallic character.
1 gold 3 sulfur 2 hydrogen 4 radon 10. Rb Mo Au B 2 See answers Advertisement Advertisement sariwodo sariwodo Answer. The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following.
QUESTION 9 Which of the following atoms has the greatest metallic character. Hence option a is correct. The elements will lose or gain electrons as needed to have.
When two elements combine to form a compound the _____ the difference in metallic character between the two elements the _____ the likelihood that the compound will be a _____ at room temperature asked Jul 29 2018 in Chemistry by Brandosem. The metallic character is the property of the metals which loses its valence electrons for forming a stable octet structure. Youll see the same procedure as you did for Figure 1 and Figure 2 as you did for Figure 1 and Figure 2.
Metallic Character The Periodic Table Of Elements

Periodic Trends Metallic And Nonmetallic Character Ck 12 Foundation
No comments for "Which of the Following Elements Has the Greatest Metallic Character"
Post a Comment